The Clinical Research Career Ladder was developed by the University to meet four goals:
- Provide progressive advancement opportunities for staff engaged in the conduction of clinical research
- Contemporize existing job descriptions for staff
- Clarify and communicate competencies or proficiencies required for each new description/level
- Ensure staff are appropriately classified, compensated and are aware of training and proficiency requirements
The ladder consists of seven tracks and five levels across which clinical research staff may move throughout their career at UAB with each level expressing, through its minimum requirements, an expectation of increased expertise through the addition of relevant experience and education.
Please note — the minimum requirements do not create the business need within a clinical research area (school/department/division/center) by which one is able to promote within the ladder. The business need is created by the duties that must be conducted by staff within the area on various protocols. The process to determine which track and level of the Ladder is most appropriate for an area’s needs is initiated when that area's HR contact emails the Clinical Research Career Ladder Title Review Committee at CareerLadder@uab.edu. If additional questions arise, please contact the HR representative for your respective area.
-
Clinical Research Career Ladder Matrix
-
Administration
Level I Level II Level III Manager Director Job Code: R010047 R010048 R010049 R010050 R010046 Grade: W.G320 W.G340 W.G355 W.G390 W.G415 FLSA Status: Nonexempt Exempt Exempt Exempt Exempt Education/ Experience: High school diploma or GED required. Bachelor's degree in a related field and three (3) years of related experience required. Work experience may substitute for education requirement. Bachelor's degree in a related field and five (5) years of related experience required. Work experience may NOT substitute for education requirement. Bachelor's degree in a related field and eight (8) years of related experience required. Work experience may NOT substitute for education requirement. Master's degree in a related field and ten (10) years of related experience required. Work experience may NOT substitute for education requirement. Licensure and/or Certification: None required. None required. Certified Clinical Research Coordinator (CCRC) or Certified Clinical Research Professional (CCRP) certification preferred. Certified Clinical Research Coordinator (CCRC) or Certified Clinical Research Professional (CCRP) certification strongly preferred. Certified Clinical Research Coordinator (CCRC) or Certified Clinical Research Professional (CCRP) certification strongly preferred. -
Coordinator
Level I Level II Level III Manager Job Code: R010051 R010052 R010053 R010054 Grade: W.G333 W.G345 W.G365 W.G390 FLSA Status: Exempt Exempt Exempt Exempt Education/ Experience: High school diploma or GED required. Bachelor's degree in a related field and three (3) years of related experience required. Work experience may substitute for education requirement. Bachelor's degree in a related field and five (5) years of related experience required. Work experience may NOT substitute for education requirement. Bachelor's degree in a related field and eight (8) years of related experience required. Work experience may NOT substitute for education requirement. Licensure and/or Certification: None required. None required. Certified Clinical Research Coordinator (CCRC) or Certified Clinical Research Professional (CCRP) certification preferred. Certified Clinical Research Coordinator (CCRC) or Certified Clinical Research Professional (CCRP) certification strongly preferred. -
Nurse Coordinator
Level I Level II Level III Manager Job Code: N040005 N040006 N040007 N040008 Grade: W.G350 W.G365 W.G375 W.G400 FLSA Status: Exempt Exempt Exempt Exempt Education/ Experience: Bachelor's degree in a related field required. Work experience may substitute for education requirement. Bachelor's degree in a related field and three (3) years of related experience required. Work experience may substitute for education requirement. Bachelor's degree in a related field and five (5) years of related experience required. Work experience may NOT substitute for education requirement. Bachelor's degree in a related field and eight (8) years of related experience required. Work experience may NOT substitute for education requirement. Currently licensed as a Registered Nurse (RN) by the Alabama Board of Nursing required. Licensure and/or Certification: Currently licensed or eligible to be licensed as a Registered Nurse (RN) by the Alabama Board of Nursing required. Currently licensed or eligible to be licensed as a Registered Nurse (RN) by the Alabama Board of Nursing required. Currently licensed as a Registered Nurse (RN) by the Alabama Board of Nursing required. Certified Clinical Research Coordinator (CCRC) or Certified Clinical Research Professional (CCRP) certification preferred. Currently licensed as a Registered Nurse (RN) by the Alabama Board of Nursing required. Certified Clinical Research Coordinator (CCRC) or Certified Clinical Research Professional (CCRP) certification strongly preferred. -
Regulatory
Level I Level II Level III Manager Job Code: R010055 R010056 R010057 R010058 Grade: W.G320 W.G340 W.G355 W.G390 FLSA Status: Nonexempt Exempt Exempt Exempt Education/ Experience: High school diploma or GED required. Bachelor's degree in a related field and three (3) years of related experience required. Work experience may substitute for education requirement. Bachelor's degree in a related field and five (5) years of related experience required. Work experience may NOT substitute for education requirement. Bachelor's degree in a related field and eight (8) years of related experience required. Work experience may NOT substitute for education requirement. Licensure and/or Certification: None required. None required. Certified Clinical Research Coordinator (CCRC) or Certified Clinical Research Professional (CCRP) certification preferred. Certified Clinical Research Coordinator (CCRC) or Certified Clinical Research Professional (CCRP) certification strongly preferred. -
Data Management
Level I Level II Level III Manager Job Code: I010060 I010061 I010062 I010063 Grade: W.G315 W.G335 W.G355 W.G390 FLSA Status: Nonexempt Exempt Exempt Exempt Education/ Experience: High school diploma or GED required. Bachelor's degree in a related field and three (3) years of related experience required. Work experience may substitute for education requirement. Bachelor's degree in a related field and five (5) years of related experience required. Work experience may NOT substitute for education requirement. Bachelor's degree in a related field and eight (8) years of related experience required. Work experience may NOT substitute for education requirement. Licensure and/or Certification: None required. None required. Certified Clinical Research Coordinator (CCRC) or Certified Clinical Research Professional (CCRP) certification preferred. Certified Clinical Research Coordinator (CCRC) or Certified Clinical Research Professional (CCRP) certification strongly preferred. -
Recruiter
Level I Job Code: R010159 Grade: W.G285 FLSA Status: Nonexempt Education/ Experience: High school diploma or GED required. Licensure and/or Certification: None required. -
Quality Coordinator
Level I Job Code: R010187 Grade: W.G345 FLSA Status: Exempt Education/ Experience: Bachelor’s degree in a related field and three (3) years of related experience required. Work experience may substitute for education requirement. Licensure and/or Certification: None required.
-
Administration
-
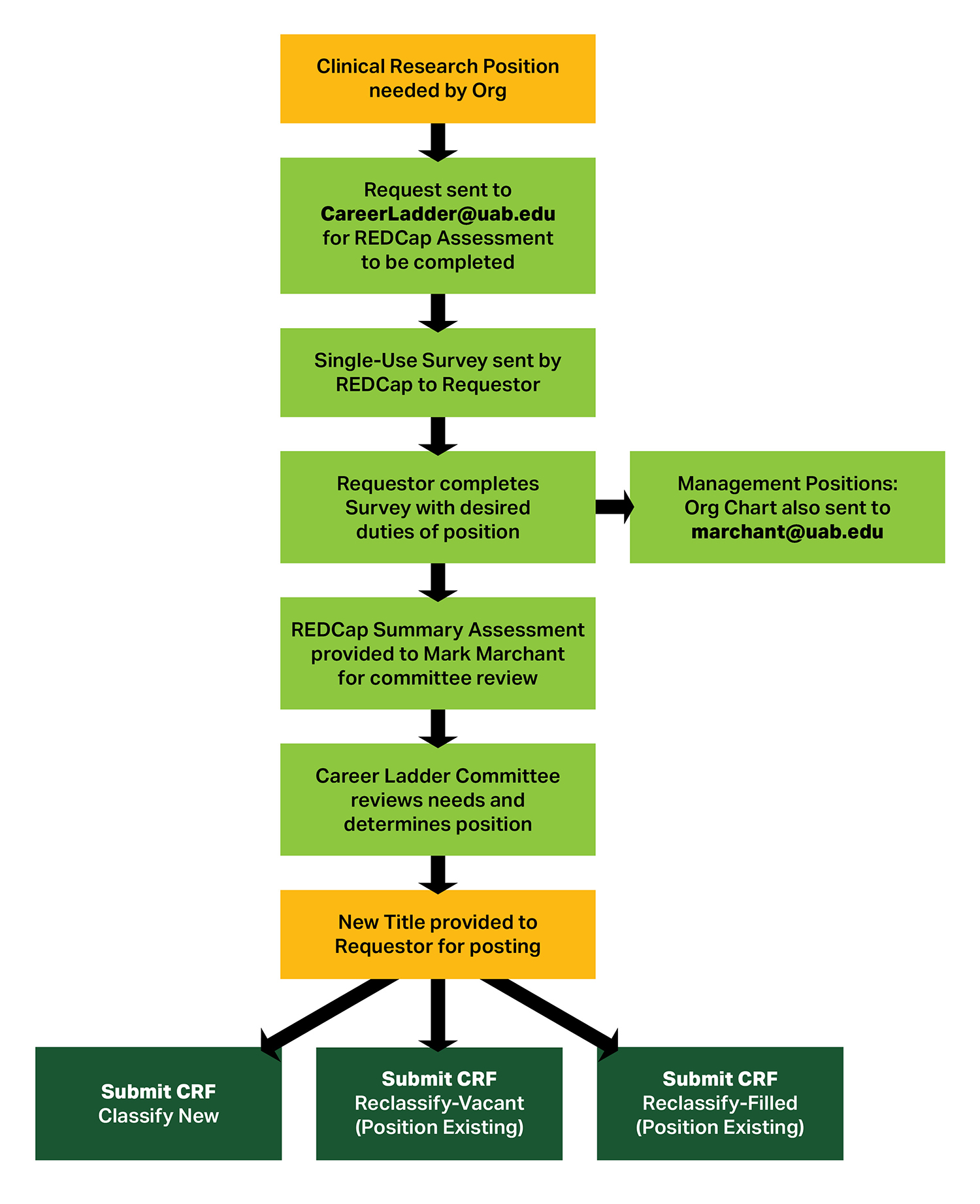
Clinical Research Career Ladder Requestor Process
Please follow the process outlined below prior to submitting requests to Compensation for positions on the Clinical Research Career Ladder.
Requestor Process Text Alternative
-
Pay Ranges
Grades for jobs in the Clinical Research Career Ladder fall within the UAB General Structure, which can be found in the Pay Structures section of the Compensation website. Please refer to the current fiscal year document for the associated pay grades and assigned pay ranges.